Since the last session in 2018, the MiFoBio regulars were waiting for its return after Covid-19. After three years of absence, MiFoBio multidisciplinary school has gathered again from 5 to 12 November 2021, in South of France.
Each two-years, for the past 15 years, MiFoBio (Functional Microscopy for Biology) reunites microscopists from France, Europe and North America. This school is organized by the CNRS (National Center for Scientific Research) through the ImaBio Research Group. It focuses on biological and technological advances for life imaging and quantification.
This very intense session has seen 390 attendees, 55 speakers, 56 industrials with 103 representants, 127 workshops, 10 round tables, 46 courses, 10 seminars and 4 pre-modules. On topics such as quantification in nanoscopy, multicellular imaging, labelling, probes and contrast, or AI for bioimaging.
In our discussions with the participants, some trends did appear: this year, the topic of Structured Illumination Microscopy was more represented in the discussed thematics, as well as deep learning. Two topics that certainly have been growing in the past years.
Argolight was there to help microscopists to provide the tools to microscopists to verify their system before their workshop.
Round table: “Quality monitoring of a photonic microscope over time: talk with manufacturers”
During this edition, a Round Table has been organized by the initiative of the metrology group of the RT-mfm (Technology network for Multidimensional fluorescence microscopy). It gathered manufacturers, such as Leica, Nikon, Olympus and Zeiss, and microscopists, to discuss the needs of the community in terms of quality monitoring. Discussion targeted the approaches available and proposed by the RT-mfm to follow within time the performance of fluorescence microscopes, a topic on which manufacturers are still hesitant. Providing users with metrics that would apply to everyone is still an uncomfortable matter.
1. Workshops on Metrology
Courses and workshops of MiFoBio are known to be qualified training sessions. We list here some of the workshops where an Argolight slide has been used.
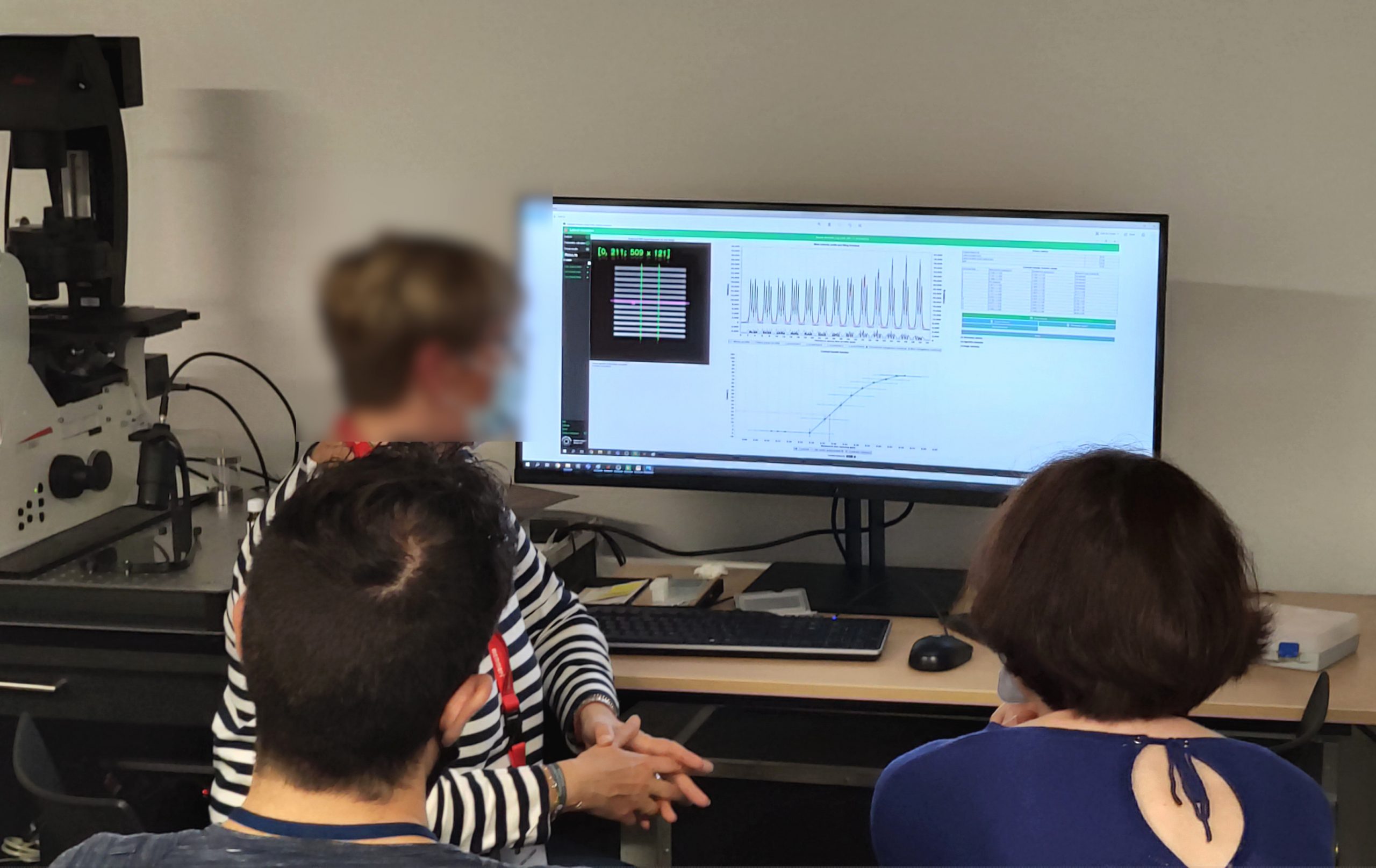
High resolution wide-field microscopy and digital processing on a thick sample
Lead by: Vicky Diakou-Verdin, from Montpellier University Imaging core
Argolight slide used: Argo-HM v2
Abstract: MiFobio Program
In wide-field microscopy, more and more manufacturers are integrating image processing algorithms into their acquisition software in order to improve the quality of the images produced (improvement of the resolution and of the signal-to-noise ratio, noise reduction, deblurring). These post-acquisition processing uses often complex algorithmic approaches such as deconvolution, Wiener filters, wavelet approaches, etc.
The objective of this workshop is to test whether these techniques combining wide-field microscopy and image processing can be used for all types of samples. What are their advantages and limitations? Are the images then quantifiable? Can these techniques compete with more classic signal enhancement approaches (confocal imaging, deconvolution for example)? Can we go back to “basic” microscopy?
We will observe at different scales several types of fixed samples (mammalian cells, Drosophila embryos, plant roots) which will lead us to find the advantages and the limits of these techniques. We will see how to configure and optimize post-acquisition processing and we will be able to compare different techniques. We will also use methods and tools to ensure that the techniques used are quantitative.
At the end of the workshop, participants will be able to determine if wide-field microscopy combined with an image processing method can be an acceptable alternative to more conventional microscopies such as confocal imaging; and what types of samples are compatible with these approaches.
Illumination power live measurement and power fluctuations observation, displayed by Daybook software during a workshop, using the power meter of an Argo-POWERHM.
Through the “Lateral resolution” and “Intensity response” analyses in Daybook software, the participants could observe the consequences of different “computational clearing” methods in terms of contrast, SNR and resolution improvement, as well as intensity ratio conservation.
Imaging multiprotein complexes in the cytosol by super‐resolution fluorescence. Introduction to lattice and dual iterative SIM
Lead by: Elodie Chatre and Stanimira Valeva, from Imaging and Microscopy Core Facility (PLATIM)
Argolight slide used: Argo-SIM v1
Abstract: MiFobio Program
Structured illumination microscopy (SIM) is a technique that doubles the resolution typically obtained in confocal microscopy as SIM imaging allows to reach lateral resolution of 100‐130 nm (1,2) and axial resolution of 280‐350 nm (3,4). Multicolor 3D image acquisition in SIM is fast, can be done on living or fixed samples and is compatible with most chemical or biological stains. Thus, SIM can be particularly useful for studying cellular structures, dynamics and/or interactions on a cellular or tissular level. The purpose of this workshop is to present the SIM methodology and the functionalities of the lattice SIM system of the Elyra 7 microscope from Zeiss. We will also introduce the new processing method developed by Zeiss – deconvolution applied to SIM, called diSIM (dual iterative SIM) or SIM². Finally, this workshop will give to the participants the opportunity to evaluate the usefulness of SIM for the needs of their projects.
Using the “Gradually spaced lines” pattern of an Argo-SIM, it could be demonstrated that the lateral resolution of a Zeiss Elyra 7 with Lattice SIM was 120 nm (63x objective, 1.46 NA, 405nm). In similar acquisition conditions, with the new diLattice SIM (or SIM 2), 60 nm resolving power could be reached.
Probabilistic pipeline to extract reliable information from single molecule microscopy data
Lead by: Al Hassan Cassé from the histopathology and Bio-Imaging Lab at Sanofi, and Jean-Baptiste Masson, from Computational biology department at Pasteur Institute
Argolight slide used: Argo-POWER v1
Abstract: MiFobio Program
Tumor necrosis factor receptors (TNFR) and their interacting ligands are crucial for immune regulation, cell proliferation, survival, and death. They have demonstrated ability for treating infectious disease, autoimmunity, and cancer. TNFR has been shown to be capable of enhancing TCR‐dependent activation of both CD4 and CD8 T cells, inducing increase of IL‐2 receptors levels, enhancing T cell proliferation and cytokine production. TNF superfamily (TNFSF) ligands and their receptors have several functional and structural characteristics implicated in cell signal transduction and protein recruitment. To trigger signaling, the binding of trimeric ligand to three monomers receptors is necessary, creating hexagonal network of TNF‐TNFR. Single molecule microscopy can achieve 10nm of resolution, allowing the visualization and a precise analysis of checkpoint receptors dynamic and interaction. Since little is known about how really the checkpoint receptors interact each other in single or double activation. Cambulac will help us to elucidate those interaction and determine which multispecifics mAbs structure gave the best T cells immune response. Both T cells CD4, CD8 and T regulatory will be screened using Bayesian inference and deep learning approach. Cambulac will also help in other projects where complex molecules binding, and interactions are involved. These unique statistical tools to compare complex experiment at single molecule scale will be an added value to accelerate and unravel unsuspected molecular interactions on cell surface.
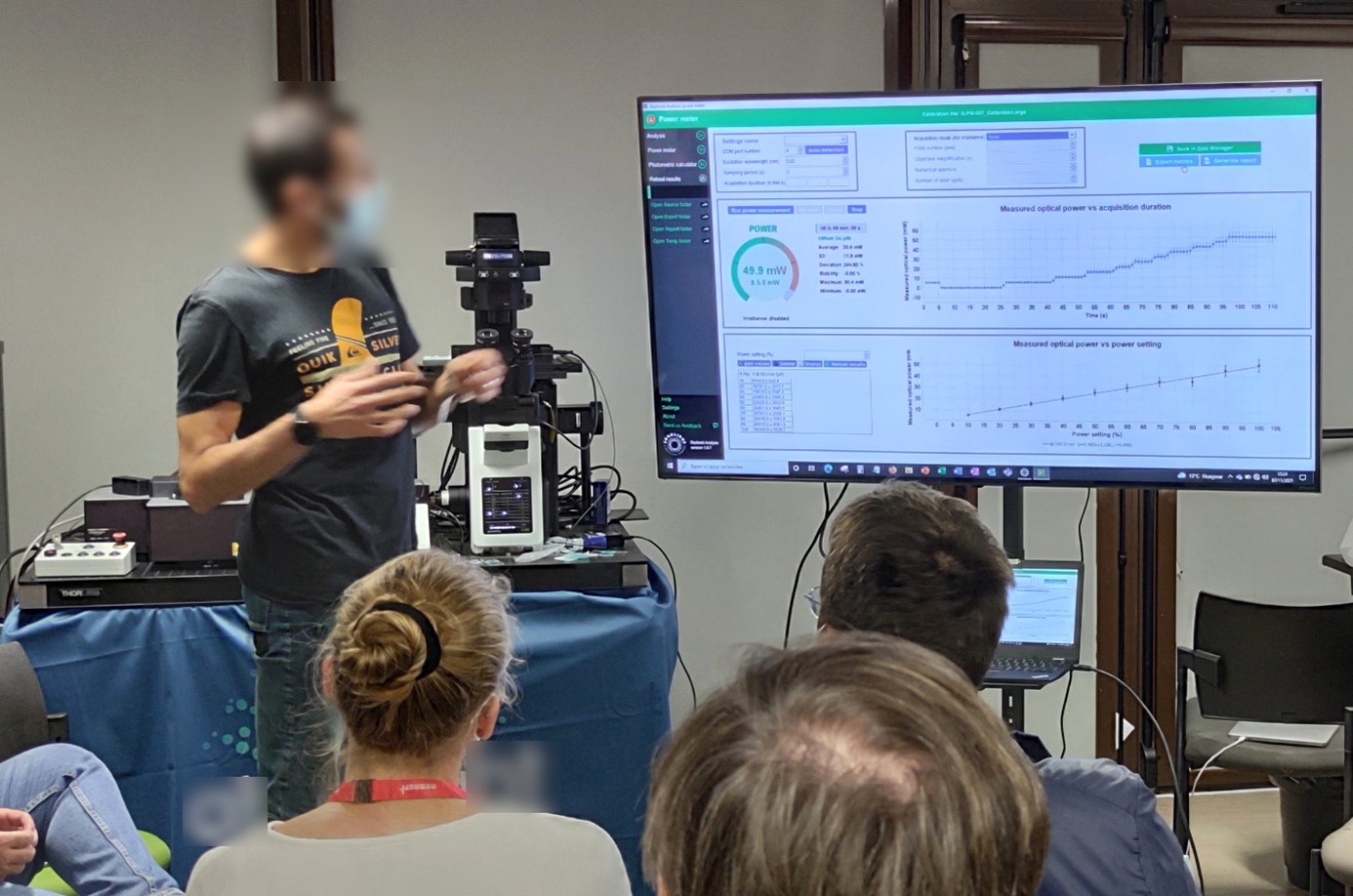
The illumination source of a microscope impacts greatly the behavior of cells and can even induce phototoxicity. Three factors are to be considered: the optical power, the exposure time, and the illumination wavelength.
During the workshop, the Sanofi-Pasteur team explained how to track their system’s light source performance over time, to prevent their decline or abnormal behaviors.
The power meter of an Argo-POWERHM has been used to measure in real time the illumination power and demonstrate the linear relationship between the power setting (in %, set on the microscope) and the measured value at the sample location (in Watts).
The necessity to contextualize the data acquisition conditions is increasingly recognized by the scientific community. Running metrology on microscopy instruments is one way to ensure the relevance and quality of the data obtained.
The necessity to contextualize the data acquisition conditions is increasingly recognized by the scientific community. Running metrology on microscopy instruments is one way to ensure the relevance and quality of the data obtained.
Illumination power live measurement and power fluctuations observation, displayed by Daybook software during a workshop, using the power meter of an Argo-POWERHM.
2. Optic Lab: basis of microscopy measurements
The OpticLab has been an essential addition to this MiFoBio session. Animated by expert microscopists, it aims at sharing the best ways to make measurements on optical systems through practical sessions. Both beginners or more experienced microscopists can find interest.
They are “organized as four “Paths”, each comprising stations with optical benches, to demonstrate the principles of optics (part “Optics in practice & Manufacture of images”), and stations with microscopes, to learn practical daily applications, including maintenance and setting up the microscope, diagnosing problems, etc. (“Applications” section).” [1]
To run this session, the RT-mfm provides metrology tools, selected from the Metrology Case. The metrology case is an initiative from the RT-mfm to gather the necessary tools to measure the performance of fluorescence microscopes, and to lend them to their network.
These hands-on courses are a good way to get the basics in quality control:
- Settings of a microscope: it aims at performing an alignment to get a Köhler illumination and verifying the importance of this within image acquisition in transmitted light.
- Vignetting and field homogeneity: it aims at understanding the role of light source alignment in the image acquisition; as well as measuring and analyzing the field uniformity and knowing which issues can arise.
- Measuring the Point Spread Function: It aims at preparing a slide with fluorescent beads, whose size are smaller than the resolution of the microscope, in order to acquire and measure a PSF. Participants also observe the role of the refraction index in the mounting and immersion medium.
- Alignment and corrections: it aims at measuring the shift between two channels, due to the objective (chromatic correction), to dichroic mirrors or to cameras; as well as showing that alignment is never perfect.
Once again, this school has been a great event to discuss quality control with microscopists and Argolight users. For those of you who could not attend the live event, some presentations are available in replay on YouTube. You can watch them here:
5 November afternoon https://www.youtube.com/watch?v=PLqlovXNeq0
6 November morning https://www.youtube.com/watch?v=7H_kUNtujCI
6 November afternoon https://www.youtube.com/watch?v=OfXXk5W67MY
7 November morning https://www.youtube.com/watch?v=alTm_X5zJ4c
7 November afternoon https://www.youtube.com/watch?v=q8SMfb4kPD4
8 November morning replay https://www.youtube.com/watch?v=cwCJz3Y4FU4
8 November afternoon https://www.youtube.com/watch?v=SKGTRncESxs
9 November afternoon replay https://www.youtube.com/watch?v=cwCJz3Y4FU4
10 November morning https://www.youtube.com/watch?v=b33PbrJx30k
10 November afternoon https://www.youtube.com/watch?v=6kI04YqYVQY
11 November morning https://www.youtube.com/watch?v=shsDO4g9lQo
11 November afternoon https://www.youtube.com/watch?v=GLCOSjE_4iY
See you there in two years!
[1] MiFoBio OpticLab leaflet http://imabio-cnrs.fr/wp-content/uploads/2021/11/OpticLab_MIFOBIO2021.pdf?x88135
User case: Kyoto University x Argolight – Fluorescent proteins observation
Matsuda Lab, Graduate School of Biostudies/Graduate School of Medicine, Kyoto University Introducing Argolight slides - A New Quality Control...
Quality control of HCS-HTS fluorescence imaging systems
In the landscape of high-content screening (HCS) and high-throughput screening (HTS) fluorescence imaging systems, precision and reliability take...
Precision Partners: Innopsys and Argolight on the InnoQuant Slide Scanners
In the intricate realm of pathology, drug discovery, and advanced research in brain function, cancer, and stem cells, the role of slide scanners has...