1. How often should we perform Quality control?
Many factors will determine how often the quality of a fluorescence microscope should be controlled:
- the type of instrument (widefield versus confocal): confocal systems are much more complex than widefield systems, and they have more components that could deviate.
- its components: mercury or metal-halide lamp versus more stable LEDs,
- the experience of the user: an experienced microscopist will notice changes more readily than a novice,
- whether the system is shared: “in a multiuser environment, microscopes are prone to contamination, misalignment or even damage of optical components.” [1]
- the type of experiment for which it is used,
- the level of quality needed: some studies might require more accuracy.
“A regular evaluation of the performance of a CF is prerequisite for maintaining high standards of operation and quality of the scientific work.” [2]
It may not be realistic to control all the above-mentioned aspects before each imaging session. However, depending on the study, some of them need to be assessed.
Simple examples are:
- the control of the objective status before any imaging session,
- the control of chromatic aberrations before colocalization studies,
- the control of the system’s intensity response before any experiment where intensity is meant to be used.
A deeper control can take place just after the installation of a new system or after a maintenance[3]. A reference status of the system performances can then be defined: a point to go back for comparison if needed, each time the system is assessed.
Thus, manufacturer specifications can be validated, and system’s performances monitored over time.
“When performed regularly, standardized test series often help identify damaged and misaligned microscope components, even before they significantly impair the quality of user data." [4]
2. How often is Quality control really performed?
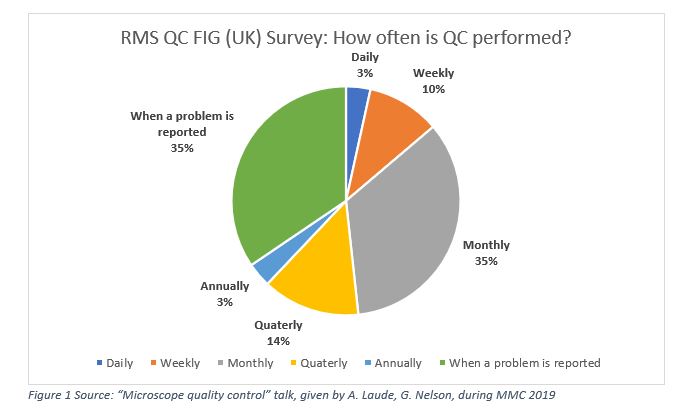
The RMS Quality Control Focused Interest Group (RMS QC FIG) presented a talk [5] at MMC in 2019: in a survey conducted in UK on 29 respondents, ~1/3 were performing QC every on a monthly basis, and ~1/3 when a problem was reported.
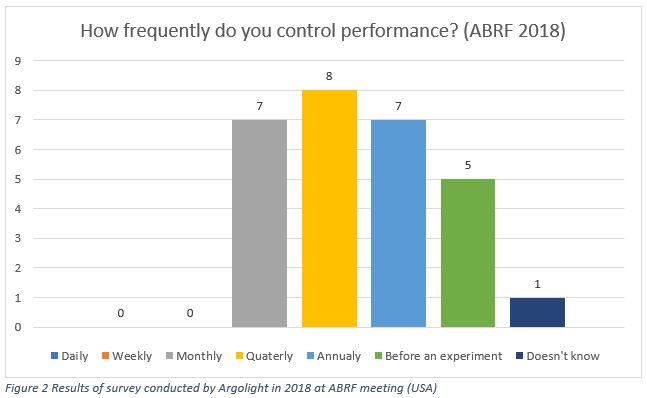
In a 2018 survey conducted by Argolight on core facility managers during ABRF conference in the USA, the results were very much spread out.
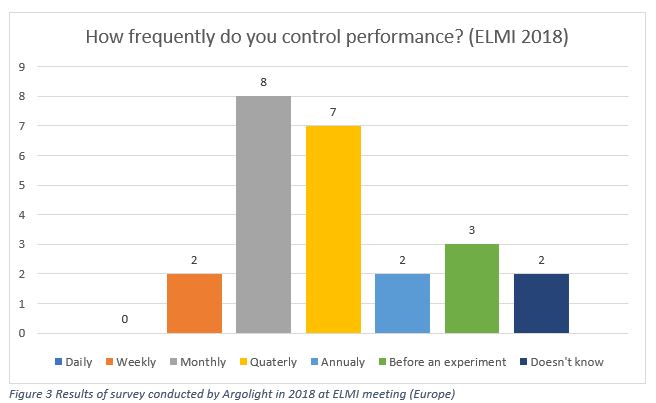
The same survey, conducted by Argolight during ELMI 2018 in Dublin, Ireland, gave different answers.
During our webinars in 2020, we asked the participants “How frequently do you (yourself or your colleagues) perform QC session?”. The majority of the 38 respondents (mainly Europe) answered that they had 1 or 2 Quality control sessions per month. Other answers: ~1 per week: 11%; ~1 or 2 per month: 42%; ~1 per quarter: 26%; ~1 per semester: 13%; ~1 per year or less: 8%
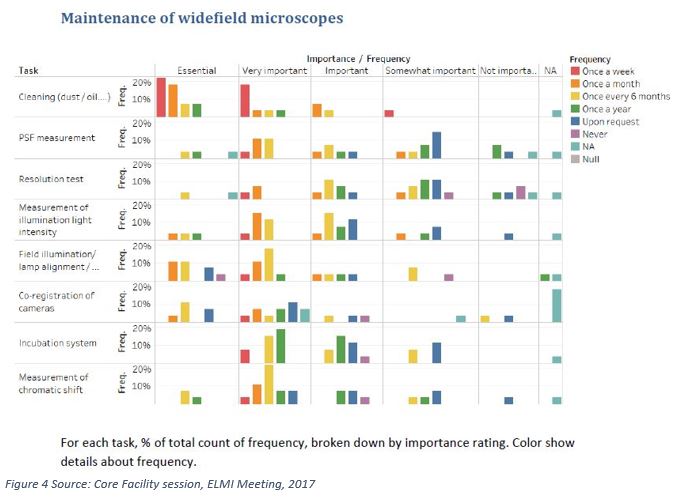
In the 2017, the European Light Microscopy (ELMI) meeting included a core facility session, where a survey on microscope maintenance was conducted [6]. Respondents were asked to tell how often they would control some measurement on their widefield microscopes and on their confocal microscopes, rating it from once a week to never.
A large survey for Core Facilities (200+ answers from Light microscopy, EM and Image Analysis) has been put together for the European Light Microscopy (ELMI) meeting in 2019 and is still ongoing. You can still participate.
In a 2010 article, the Friedrich Miescher Institute worked on a procedure for routine assessment of fluorescence microscope performance. The writers “found that checking performance at a weekly interval was a good compromise between workload and reaction time to spot performance flaws on systems that are used for more than 1500 hours per year.”[7]
“Checking performance at a weekly interval was a good compromise between workload and reaction time to spot performance flaws on systems that are used for more than 1500 hours per year."[7]
3. In a nutshell
Assessing periodically a fluorescence microscope enables to quickly identify and solve microscope issues. This would prevent the acquisition of corrupted data, and the consequent question: since when the data generated by the microscope has been corrupted? It also minimizes machine downtime by optimizing maintenance, not based on a subjective image of a biological sample, but on objective and quantified parameters.
Each facility will design its own schedule and routine to work within the constraints and provide the best service possible. The easier and the more automated QC tools will be, the less constraints there will be to perform QC.
This article’s content is extracted from our Applications guide https://argolight.com/files/Argolight-solutions_Applications-guide.pdf
Sources:
[1] [2] [4] E. Ferrando-May et al.., Microscopy Research and Technique 79, p469 (2016).
[3] « Understanding light Microscopy », J. Sanderson, chapter 27.6, p648
[5] “Microscope quality control” talk, given by A. Laude, G. Nelson, during MMC 2019
[6] ELMI 2017 core facility session, Results of the Survey on microscope maintenance, Page 3 and 4, https://elmi.embl.org/wp-content/uploads/2017/05/Maintenance-Results_ELMI-CF_session2017.pdf
[7] « Routine Assessment of Fluorescence Microscope Performance, An ImageJ Macro to Speed Up PSF Display and Analysis », L. Gelman, J. Rietdorf, (2010), DOI: 10.13140/RG.2.1.3977.1604
Header photo by Nathan Dumlao on Unsplash
User case: Kyoto University x Argolight – Fluorescent proteins observation
Matsuda Lab, Graduate School of Biostudies/Graduate School of Medicine, Kyoto University Introducing Argolight slides - A New Quality Control...
Quality control of HCS-HTS fluorescence imaging systems
In the landscape of high-content screening (HCS) and high-throughput screening (HTS) fluorescence imaging systems, precision and reliability take...
Precision Partners: Innopsys and Argolight on the InnoQuant Slide Scanners
In the intricate realm of pathology, drug discovery, and advanced research in brain function, cancer, and stem cells, the role of slide scanners has...