Using fluorescent plastic slides to measure field uniformity
ReadYou may be familiar with the fluorescent plastic slides that are handed out during trade shows.
As they are easy to use, they are often employed to measure the field uniformity of a fluorescence microscope. If they can be useful in some cases, they are not the best tools to measure the field uniformity of a fluorescence imaging system, as some of their features prevent using them within a quantitative approach.
1. Why measuring the field uniformity?
Many applications in fluorescence microscopy base their conclusions on the quantity of signal exhibited in the images. But often, even if the sample has an even intensity across the field of view, some areas of its image appear brighter than others. This local variation of the measured intensity is also known as “shading”, “field uniformity”, “homogeneity”, “evenness”, or “flatness”. This effect can come either from the illumination or from the detection paths.1 The latter will not be discussed in this document.
For example, typical roll-off values, i.e. the normalized intensity drop over an intensity line profile along a diagonal, range from 20 to 40 % for confocal fluorescence microscopes. Lower than 10 % would be ideal, though it seems difficult to be attained today 2.
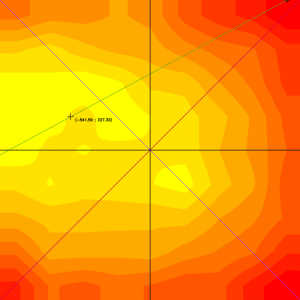
Figure 1: Example of a heat map showing the uniformity of a field of view. The maximum of the illumination is not perfectly centered: it is slightly decentered towards the top left. The minimum of the illumination is close to the top right corner of the image.
More broadly, in any fluorescence microscope, without a dedicated module inside the microscope, the spatial distribution of the illumination intensity is never perfectly homogeneous. It is usually maximal in the center of the field of view (if the microscope is well-aligned) and decreases towards the edges. This results in a biological sample that is not homogeneously illuminated.
If not considered, this shading effect can impact the conclusions of the experiment. The underlying assumption that there is a correlation between fluorescence intensity in the image and fluorophore concentration in the sample is not true anymore.
The knowledge of the spatial distribution of the illumination intensity onto a sample is therefore important to prevent from misinterpreting intensity differences in images of biological samples.
Both ISO 21073:2019 and ASTM F3294 standards define field uniformity as one of the parameters to be measured in a fluorescence microscope and eventually corrected trough post-acquisition image processing.
2. What are the fluorescent plastic slides? What are their advantages? What are they made for?
- What are the fluorescent plastic slides?
Fluorescent plastic slides are made of bulk fluorescent polymer material, emulating a thick and continuous fluorescent sea. They come in different colors, depending on the material doping the polymer they are made of. They exhibit different excitation and emission wavelengths. They are mostly known to be produced by Chroma, but similar products are available from Ted Pella or Thorlabs. 3

Figure 2: Example of fluorescent plastics slide, here from Ted Pella.4 Because of their different spectral (absorption and emission) properties, they look either blue, red, greenish, or yellow.
- What are their advantages?
The advantages of these slides are clear: “made of rather durable plastic, they provide a continuous fluorescent field. No hunting for microspheres. No cellular photo-bleaching.” 4
They do not need refrigeration and can be stored directly in their box. Their fluorescence is also very bright due to high concentration of fluorescent doping material.
Their cost is also a big reason of their success: they range from inexpensive ($4 each) to free (Chroma usually provides them on their conference booths).
- What are they made for?
Fluorescence plastic slides are sold to:
– “Enable a check of how evenly a fluorescence illuminator is filling the field of view and whether it is centered.” 5
– “Assess and align fluorescence imaging systems.” 5
However, there is no consensus in using them to measure the field uniformity. On the one hand, they are not cited in the ISO 21073:2019 standard (about measuring performance data of confocal fluorescence microscopes). On the other hand, microscope manufacturers do not provide guidelines, white papers, nor tutorials on how to use these slides for this purpose. Therefore, it is hard to determine the exact usage range of these products.
"Microscope manufacturers do not provide guidelines, white papers, nor tutorials on how to use these slides for this purpose. Therefore, it is hard to determine the exact usage range of these products."
3. Are there limitations to this tool?
The limitations of a tool/standard within the context of quality control are of two types:
– Documentary: to perform good quality control, one must use tools that demonstrate reproducibility in fabrication quality and/or traceability to a known standard via documentation.
– Technical: the technical specifications of a standard determine the limitations on how to use it and what for.
Documentary
From a metrological and quality point of view, it is necessary to get precise, traceable, and warranted technical specifications to give meaning to the results.
This type of information clearly states the engagement that the manufacturer makes toward the client in terms of quality. Knowing which specification can be relied upon when using a sample is necessary to build a quality control approach.
To the best of our knowledge, fluorescent plastic slides are not provided with values of fluorescence stability over time, variation from slide to slide, and most importantly for the given application, homogeneity over the surface (and depth) of the slides.
Technical:
- Thickness
When using a multi pinhole system (e.g. spinning disk, iSIM, Airyscan / SPAD arrays), you do not want to accentuate the pinhole cross-talk effect.
The cross-talk effect is the effect of light originating from remote focal planes passing through adjacent pinholes to create a haze level that obscures the sharp specimen detail normally observed in confocal microscopy.
Plastic slides are 1-mm thick. Their thickness accentuates this effect, due to their intense brightness that extends well beyond the focal volume.
- Inhomogeneity
Inhomogeneities within any fluorescent material related to the chemical composition or the depth alter intensity measurements. Since no information is provided about the homogeneity of the plastic slides, field uniformity measurements obtained with such tools should be taken very carefully.
- Photo-bleaching
The photo-bleaching occurring within some fluorescent materials alter the intensity measurements. This applies to plastic slides. This can be an issue over time, as a previously photo-bleached area are drowned and invisible in the fluorescent sea. It is therefore advised to image a different/fresh area of the slide for each new measurement. Otherwise, an already bleached area of the slide can be part of the analyzed field of view 6.
- Refractive index
Because of their lower refractive index (~1.45) compared to microscope coverslips (~1.52), plastic slides introduce spherical aberration.7 This artificially minimizes the amount of intensity roll-off in the field uniformity measurements.8 The suitable tools to measure field uniformity should have a microscope coverslip on top of them, to be compatible with objective lenses designed to work after a coverslip.
- Brightness
Plastic slides emit a strong signal. Therefore, illumination power and/or detector settings must be adjusted to very low values, very different from usual biological specimens. This can impact the assessment of the imaging system as the field uniformity of the microscope might be different depending on the intensity emitted by the sample.
For example, detectors may not react the same way when hit with low or high intensities. It is advised to use a tool that emit similar amounts of fluorescence than your biological samples.9
4. Conclusion
As a bulk material providing a continuous fluorescent field, plastic slides are quick and easy-to-use tools to coarsely assess and align a microscope.
When used for measuring the field uniformity, the physical and chemical features of the fluorescent slides introduce quite a lot of deviation. It makes them inappropriate for this type of measurement.
Other tools, described in the scientific literature and in ISO and/or ASTM standards, would be suitable alternatives for this purpose.
5. Additional material: Comparisons of a dye sample and a fluorescent slide
Considering the dye solutions are recommended by the articles quoted above, it is interesting to see how plastic slides behave in comparison.
Figure 3 shows results obtained on a confocal LSM 710 for two zoom values (1 and 0.6) with a fluorescent plastic slide and a dye solution at saturation (fluorescein) sandwiched between a slide and a coverslip.
For the EYFP channel, the intensity distribution behavior looks similar for both plastic slide and dye solution, although the roll-off values are higher for the latter. For the ECFP channel, the intensity distribution behavior is quite different. Roll-off values are much more important for the dye solution.
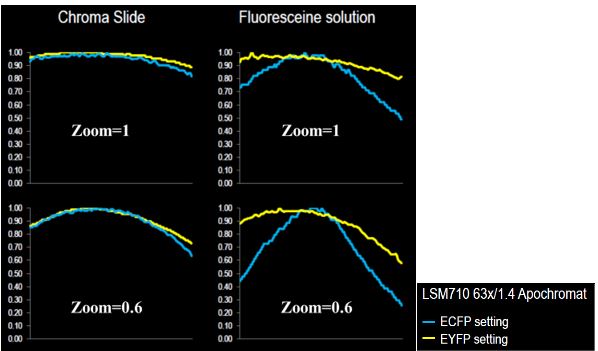
Figure 3: On the left, the intensity profile of one diagonal of the intensity distribution map obtained from a plastic slide; on the right, the intensity profile of one diagonal of the intensity distribution map obtained from a highly concentrated fluorescein solution; both for ECFP and EYFP channels. Courtesy of Laurent Gelman, Friedrich Miescher Institute, Basel, Switzerland
Related terms: illumination, field uniformity, evenness, flatness, roll-off, shading
Sources:
(1) https://www.youtube.com/watch?v=Fn8Q5AYusOI from 7.42. Talk by Spectral Applied Research “Methods to measure and correct non-uniform fluorescence illumination”, John Oreopoulos, 2011
(2) « It would be helpful if the imaging community (both users and manufacturers together) would adopt a standard requiring 90% illumination uniformity at zoom 1, measured according to the recently created ISO standard for CLSMs”. From “Tutorial: guidance for quantitative confocal microscopy”, J. Jonkman, C.M. Brown et al., Nat Protoc 15, 1585–1611 (2020), p.18. https://doi.org/10.1038/s41596-020-0313-9
(3) https://www.chroma.com/products/accessories/92001-autofluorescent-plastic-slides
(4) https://www.tedpella.com/histo_html/fluor.htm.aspx
(5) https://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=12142
(6) “Autofluorescent plastic slides tend to be hard to use as a reference: first, the absence of a glass/material interface makes it hard to set a precise focus; second, the material tends to bleach under mid-to-high illumination powers.” From “Which Elements to Build Co-localization Workflows? From “From Metrology to Analysis”, P. Mascalchi, F.P. Cordelières, Chapter 10, p. 208, In: Rebollo E., Bosch M. (eds) Computer Optimized Microscopy. Methods in Molecular Biology, vol 2040, (2019), doi.org/10.1007/978-1-4939-9686-5_10
(6) « When dealing with immersion objectives, the experimenter should also bear in mind the need for a proper cover glass to be mounted onto the slide. » From “Which Elements to Build Co-localization Workflows? From “From Metrology to Analysis”, P. Mascalchi, F.P. Cordelières, Chapter 10, p. 208, In: Rebollo E., Bosch M. (eds) Computer Optimized Microscopy. Methods in Molecular Biology, vol 2040, (2019), doi.org/10.1007/978-1-4939-9686-5_10
(7) https://www.youtube.com/watch?v=Fn8Q5AYusOI at 6.50. Talk by Spectral Applied Research “Methods to measure and correct non-uniform fluorescence illumination”, John Oreopoulos, 2011
(8) “Imaging of the uniformly fluorescent reference sample must be performed under the same imaging conditions as the sample, since any change in the optical path, spectral filters, and detection sensor may change the flatfield correction.” From ASTM F3294 − 18 Standard Guide for Performing Quantitative Fluorescence Intensity measurements in Cell-based Assays with Widefield Epifluorescence Microscopy
User case: Kyoto University x Argolight – Fluorescent proteins observation
Matsuda Lab, Graduate School of Biostudies/Graduate School of Medicine, Kyoto University Introducing Argolight slides - A New Quality Control...
Quality control of HCS-HTS fluorescence imaging systems
In the landscape of high-content screening (HCS) and high-throughput screening (HTS) fluorescence imaging systems, precision and reliability take...
Precision Partners: Innopsys and Argolight on the InnoQuant Slide Scanners
In the intricate realm of pathology, drug discovery, and advanced research in brain function, cancer, and stem cells, the role of slide scanners has...


