Medical diagnostics certification
How to secure the quality control of a new method?The laboratory of Hematology of Marseille hospitals (Assistance Publique Hopitaux de Marseille, AP-HM), located in Marseille, France, has been working on a clever project of innovative medical diagnostics. They applied for accreditation through the COFRAC, the French accreditation committee.
Audrey, Customer Care Manager at Argolight:
You have an innovating project of medical diagnostics, which is being developed in about ten facilities in Europe only. Can you tell us more?
AP-HM:
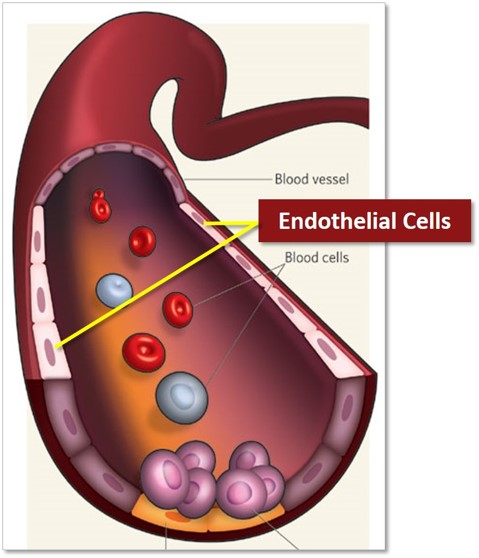
The circulating endothelial cells (CECs), described for the first time by our laboratory (1,2), are mature cells detached from the vascular wall and present in the peripheral blood. We have developed an immuno-magnetic detection method that is currently recognized as a reference method (3). The advantage of this approach is to give non-invasive access to the endothelium, whose exploration is relevant in many pathological situations (thrombosis, inflammation, transplant rejection, cancer) but difficult to achieve to date. CECs detection also helps in the diagnostic of many more pathologies, read more below**.
“We have developed an immuno-magnetic detection method that is currently recognized as a reference method.”
At the national level, we have coordinated the integration of this analysis within the framework of innovative acts outside nomenclature (Référentiel des actes Innovants Hors Nomenclature, RIHN), and on a larger scale, within the framework of the activities of the International Society of Thrombosis and Haemostasis (ISTH). Between 2004 and 2014, we set up and animated a vascular biology standardization subcommittee, gathering a small panel of experts focused on the nomenclature and determination of these cells.
Audrey:
This project requires a certification from the COFRAC, the French accreditation committee. Becoming certified is not always an easy task…
AP-HM:
Today, our laboratory is applying for validation of the Ministry of Solidarity and Health to become a reference Laboratory of Medical Biology for a number of highly specialized Vascular Biology examinations including this CECs analysis. This application requires the accreditation of the laboratory by the COFRAC for all of these analyzes.
The requirements for a COFRAC accreditation are numerous. The accreditation file includes the verification of the method performance (repeatability, reproducibility, precision, accuracy, measurement uncertainty, etc.) as defined by the manufacturer and the National Quality Control bodies. It also includes the control of the risks linked to Matter (sample), Medium, Material (reagents and equipment), Labor (staff), and Method.
For a very specialized analysis, such as CECs, the procedure is even more complex. As it is developed in-house, it does not consist solely in verifying the method performance but also in establishing the quality criteria of such a method, in order to demonstrate these criteria are controlled and validated.
“To our knowledge, there were no existing regulations to guide a laboratory wanting to establish this quality control.”
Audrey:
You called on Argolight products. Why?
AP-HM:
The control of the equipment risk is reflected by the respect of external and internal maintenance schedules and in the metrological monitoring of the instruments involved in the method. For the CECs, a problem arose: the metrological monitoring of the fluorescence microscope involved in the characterization and the count of events retained by the immuno-magnetic sorting.
We needed to implement quality control to check the stability of the fluorescence lamp and to ensure the correct visualization of our fluorescent events. To our knowledge, there were no existing regulations to guide a laboratory wanting to establish this, and no department in our institution had already accredited an analysis requiring metrological monitoring of a fluorescence microscope.
The Argolight company, identified by the metrology managers of our institution, was an interesting candidate for the implementation of the metrological monitoring of our fluorescence microscope by proposing fluorescent patterns slides made stable over time.
Audrey:
In the end, how did Argolight answered to this need?
AP-HM:
Argolight provided us two Argo-Check Intensity slides and came to visit us on-site to check the compatibility of our fluorescence microscope and their technology. The results being positive, Argolight offered us a “slide + software” control system adapted to our needs.
This offer came with on-site training with documentation, and technical support, including results analysis and interpretation advice for one year. After Argolight had solved a problem of compatibility between our microscope’s images and the latest version of their Daybook analysis software, we were able to set up a metrological monitoring of our instrument, that is fast to perform, and that responds to the equipment risk that we had identified for the validation of the CEC analysis.
“We were able to set up a metrological monitoring of our instrument, that is fast to perform, and that responds to the equipment risk that we had identified for the validation of the CEC analysis.”
Audrey:
From your experience, what would be the right approach to implement the quality control process of a fluorescence microscopes?
AP-HM:
The regulation around metrological monitoring of fluorescence microscopes being not very precise, the establishment of a control system already seems in itself a guarantee of quality. For the laboratory, it nevertheless appears necessary to clearly define the critical parameters of the fluorescence microscope (resolution, homogeneity, fluorescence intensity, etc.) that may lead to the alteration of the analysis result in order to identify the appropriate metrological solution and to find a manufacturer able to answer it.
Once these parameters have been identified, all the factors that may lead to the variation of the critical parameters must be included in the definition of the authorized thresholds of the used instrument in order not to set stability requirements that are too difficult to meet and above all too strict compared to the drift risk of the result.
** This approach identifies the measurement of CECs within the peripheral blood in the “liquid biopsies”, as well as the circulating tumor cells, with all the potential brought by these strategies in the follow-up of the evolution of the pathologies and their therapeutic treatment. Defined as a marker of the endothelial lesion (4), CECs provide diagnostic assistance for acute coronary syndromes (5) and have a prognosis value in thrombotic microangiopathy (6) or coronary artery disease (7). They also make it possible to evaluate the vascular toxicity of immunosuppressive (8) or anti-tumor treatments. Another indication of their measurement is the diagnosis and monitoring of pediatric pulmonary arterial hypertension (9).
Sources:
(1) Dignat-George et al (1992) Rapid isolation of human endothelial cells from whole blood using S-Endo1 monoclonal antibody coupled to immuno-magnetic beads: demonstration of endothelial injury after angioplasty.Thromb Haemost. 1992 Jan 23;67(1):147-53.
(2) Lefevre, P., George, F., Durand, J.M., Sampol, J. (1993) Detection of circulating endothelial cells in thrombotic thrombocytopenic purpura. Thromb Haemost 69, 522.
(3) Woywodt, A., etl. (2006). “Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol.” J Thromb Haemost 4(3): 671-7.
(4) Mutin, M., Canavy, I., Blann, A., Bory, M., Sampol, J., Dignat-George, F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood (1999) May 1;93(9):2951-8.
(5) Quilici, J., Banzet, N., Paule, P., Meynard, J.B., Mutin, M., Bonnet, J.L., Ambrosi, P., Sampol, J., Dignat-George, F. Circulating endothelial cell count as a diagnostic marker for non-ST-elevation acute coronary syndromes. Circulation (2004) Sep 21;110(12):1586-91.
(6) Widemann A, Pasero C, Arnaud L, Poullin P, Loundou AD, Choukroun G, Sanderson F, Lacroix R, Sabatier F, Coppo P, Dignat-George F, Kaplanski G; ENDO-13 study group. Circulating endothelial cells and progenitors as prognostic factors during autoimmune thrombotic thrombocytopenic purpura: results of a prospective multicenter French study. J Thromb Haemost. 2014 Oct;12(10):1601-9
(7) Bonello, L., Harhouri, K., Sabatier, F., Camoin-Jau, L., Arnaud, L., Baumstarck-Barrau, K., Ait Mokhtar, O., Roubille, F., Piot, C., Lesavre, N., Paganelli, F., Dignat-George, F. Level ofadenosine diphosphate receptor P2Y12 blockade during percutaneous coronary intervention predicts the extent of endothelial injury, assessed by circulating endothelial cell measurement. J Am Coll Cardiol. (2010) Sep 21;56(13):1024-31.
(8) Al-Massarani, G., Vacher-Coponat, H., Paul, P., Widemann, A., Arnaud, L., Loundou, A., Robert, 108.Berland, Y., Dignat-George, F., Camoin-Jau, L. (2008) Impact of immunosuppressive treatment on endothelial biomarkers after kidney transplantation. Am J Transplant 8, 2360-7.
(9) Smadja, D.M., Gaussem, P., Mauge, L., Israel-Biet, D., Dignat-George, F., Peyrard, S., Agnoletti, G., Vouhe, P.R., Bonnet, D., Levy, M. (2009) Circulating Endothelial Cells. A New Candidate Biomarker of Irreversible Pulmonary Hypertension Secondary to Congenital Heart Disease. Circulation 119, 374-81.
Header photo by Trust “Tru” Katsande on Unsplash
Quality control of HCS-HTS fluorescence imaging systems
In the landscape of high-content screening (HCS) and high-throughput screening (HTS) fluorescence imaging systems, precision and reliability take...
Precision Partners: Innopsys and Argolight on the InnoQuant Slide Scanners
In the intricate realm of pathology, drug discovery, and advanced research in brain function, cancer, and stem cells, the role of slide scanners has...
Lordil Microscopy announces the first quality control service using Argolight products.
Lordil Microscopy (lordil.fr) announces the first quality control service using Argolight products. This innovative service is offered through a...